 Introductory
Biochemistry (Chemistry 330)
Introductory
Biochemistry (Chemistry 330)
Paper Chromatography
This page contains some theory on chromatography,
and procedures for doing cylindrical and circular
paper chromatography and cellulose thin layer chromatography
(TLC) of amino acids. The materials and equipment
needed are listed at the end. You can also leave me a message,
go to my home page,
or go back to the lab
schedule.
A number of modern analytical and preparative techniques
are called chromatography ("color writing"). What they
have in common is the separation of mixed samples using the components
differing interactions with a stationary phase and a moving phase.
These phases may be solid/liquid, liquid/liquid, solid/gas, etc. In
paper chromatography, for example, amino acids can be separated because
of their differing solubilities in the water of hydration around cellulose
fibers and in solvents moving through these fibers. The relative solubilities
can be changed by altering the polarity of the solvent or its pH, which
will change the ionic state of samples. Under a suitable set of conditions,
then, each molecule in a mixture will move at a different rate over the
stationary phase and be at a unique distance from a common starting point,
when solvent movement ceases. This fact can be conveniently expressed as
a retardation factor (Rf), defined as follows:
|
Retardation Factor = Rf =
|
distance sample traveled
distance solvent traveled
|
Thus, if two samples of the same molecule (one known and the other unknown)
are analyzed under identical conditions, both will have the same Rf.
This ratio will also differ (hopefully) from the Rf for any
other molecule present, supporting the identification of the unknown. A
basic principle here is that difference is proof that
the samples are different, but identical values only support
identity, since two different structures may have the same Rf
under one set of conditions. One practical example of how this principle
is used is to understand the structure of proteins.
Proteins are polymers built from more than twenty different types of
amino acid subunits joined by peptide (amide) bonds. These amino acids
differ only in the chemistry of their side chains, which can range from
ionic to polar to nonpolar, and acidic to neutral to basic (see
below). Thus, the different properties and functions of proteins are
the direct result of different amino acid sequences. Therefore, to understand
a protein, we must first be able to determine which amino acids it contains
and the order in which they occur. To do this we must be able to separate
complex mixtures of amino acids and identify them. An early but still useful
technique for this is paper chromatography.
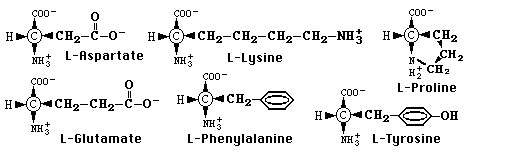
Since amino acids are not themselves visibly colored, some method of
locating them after chromatography is needed. Ninhydrin is used
for this because it reacts with free amino groups to produce a colored
product (usually purple). Several of the amino acids, however, produce
distinctive shades with ninhydrin which can aid in their identification.
Proline, for example, gives a yellow spot and tyrosine turns steel blue.
Would you like to go to the top
of this page, to my home
page, or back to the lab
schedule?
PROCEDURES:
Prepare sample applicators by heating the center of a glass capillary
in the edge of a small flame until it softens. Remove from the flame and
pull quickly and firmly. Break the fine tube to produce two microtipped
capillaries. Make enough applicators for your unknowns and standards, but
share capillaries for the standards with your colleagues to cut
waste.
Cylindrical Paper Chromatography
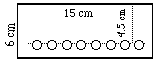
For cylindrical chromatography, you should use 2 (6 x 15 cm) sheets
of filter paper, one for solvent A and one for B. Samples should be spotted
on a line 4.5 cm from the top and 1 cm apart. Holes are punched on each
side of the samples to improve resolution. The strips will be stapled into
cylinders, and developed in 250 mL beakers with a watch glass or petri
dish as a lid.
CAUTION: Don't touch the analytical area with your fingers
(wear gloves!). They secrete amino acids, proteins, etc. which react with
ninhydrin.
Procedures:
- Add 20 mL of each solvent into a separate 250 mL beaker and cover with
watch glasses. Label them "A" or "B" to match
the solvent used.
- Obtain 50-100 µL samples of any standard amino acids in a labeled
spot plate, if desired. Share these knowns with you partners.
- Label the side of each paper with your name and the solvent to be used
(A or B).
- Draw a light line 4.5 cm below the top and make a row of light dots
0.5 cm apart on this line with a pencil.
- Punch out every other dot with the hole's center slightly below the
line and dots.
- Clamp the papers to the metal rack, if provided.
|
|
Sample Application:
- Apply 1-2 µL of each sample (2-4 applications) on (or above)
the dots selected, drying (hair drier?) between applications. The final
spots should be as small as possible for the best resolution. Apply twice
as much of an unknown.
- Dry the sample spots with a hair drier or oven.
- After sample application, pull the paper over an edge to make it curl
into a cylinder, and staple with the edges just touching, but not overlapped.
(Gloves!)
- Briefly hold each paper over a steam bath to ensure hydration of the
fibers.
- Develop as shown here and described below.
- To check procedures and colors, use a scrap strip of paper. Test the
applicators and your technique by applying samples in widely separated
and labeled positions. Then, while the analytical papers are developing,
dry the test strip, spray it with ninhydrin, and heat it to develop the
colors.
- Correct any problems before doing the developed papers.
|
|
Would you like to go to the top of this page, to
my home page,
or back to the lab
schedule?
Development:
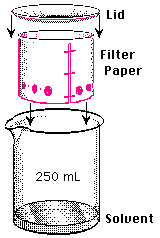
- Lower each paper into their beaker of solvent and cover with the watch
glass. Note the time. The surface of the solvent must be below the
sample spots!
- Remove the cylinder (in the hood!) when the solvent reaches the top
of the paper. (If you remove it early, mark the solvent front immediately
so you can measure for the Rf calculations.) Note the time(s).
- Dry completely (hood, then oven) until you can not detect the
odor of HAc or ammonia.
- Note the times and temperatures used.
Detection:
- Spray each paper until visibly damp, but not wet, with 0.2% ninhydrin.
- Allow to dry in the hood, and develop the colors overnight at room
temp., by heating with a hair drier or in an oven at 100°C for 5-10
minutes.
Remember:
Protect the papers from contact with your hands before, during, and
after analysis. Cover the important areas with magic tape to save them.
Protect the developed papers from light. The colors will fade with time
and exposure.
Never circle a spot; put a dot in the center of it for measuring.
Analysis:
- Measure the distance from the application point to the center of the
final spot, and the distance from the application point to the solvent
front for each spot detected.
- Calculate the Rfs of all spots on both papers.
- To identify which amino acids are present in any unknown, use Rfs
and colors.
- Remember: The key is to exclude those that
can not be present.
- Present your data in a professional manner! Label everything!
Clearly explain your reasoning for conclusions you draw!
Did you notice what happened to lysine and glutamic acid in solvents
A & B?
Explain! (Hint: What is the net charge on each in acid? in base?)
Would you like to go to the top
of this page, to my home
page, or back to the lab
schedule?
Circular Paper Chromatography
Procedure:
- Add 20 mL of each solvent, each into separate, glass petri dish lids
and cover with watch glasses. Label them "A" or "B".
- Obtain 50-100 µL samples of the standard amino acids in a labeled
spot plate, if desired. Share these with your partners.
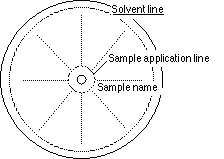
- Find the center of two 11 cm diameter Whatman #l papers.
- Scribe a sample line with a 5 mm radius, and a solvent line with a
5 cm radius.
- Push the pin through the center to enlarge the hole for a wick, and
label the side of each paper with your name and solvent letter.
- Clamp the filters to the metal rack, if provided.
|
|
Sample Application:
Apply 1-2 µL of each sample (2-4 applications) on (or above) the
dots selected, drying (hair drier?) between applications (The final spots
should be as small as possible for the best resolution). Apply twice
as much of an unknown.
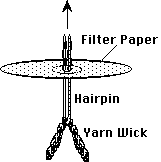
- Insert a wick of 6 cm of rug yarn, doubled over, with a flattened hair
pin, and slip it off, leaving the loop on one side of the circle and the
two ends on the other. (Wear gloves!)
- Dry the sample spots with a hair drier or oven, and briefly rehydrate
over a steam bath.
- It is a good idea to use a scrap of paper to test your applicators,
technique and reagents. Apply samples in widely separated and labeled positions.
While the other papers are developing, dry it, spray with ninhydrin, and
heat it to check procedures and colors.
|
|
Development:
|
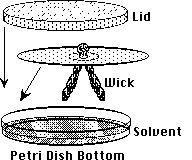
- Place the paper over the solvent with the wick down, and cover with
a watchglass or petri dish lid. Note the time.
- Remove the circle (in the hood!) when the solvent reaches the outer
line.
(If you remove it early, mark the solvent front immediately so you
can measure for the Rf calculations.) Note the time(s).
- Remove the wick by pulling the tails with a tissue (wear gloves).
- Dry completely (hood, then oven) until you detect no odor of
HAc or ammonia.
- Record the times and temperatures.
|
Detection: See above.
Analysis: See above.
Would you like to go to the beginning of the circular
paper chromatography section, to the top of this page
or back to the lab
schedule?
Alternative: Circular Thin Layer Chromatography
Cellulose TLC sheets are cut into 10 x 10 cm squares. The center is
found by laying a ruler from one vertex to the opposite one, first one
way and then the other. The pin of a compass is pushed through the backing
at the center and the other pin or pencil tip is used to gouge out the
cellulose layer in a ring with a 5 cm radius. This results in a self limiting
solvent barrier.
Other procedures are much the same as for the
paper media above. A 5 mm radius sample line is then lightly scribed
with the compass. The pin is then used to enlarge the center hole for the
wick. First, apply the samples about every 5 mm. Then, a wick of yarn,
doubled over, is inserted. The lids of glass petri dishes serve as both
solvent chambers and lids. The loop on the plastic backing side of the
TLC plate is cut off to remove the yarn wick. Development,
detection and analysis are
the same as for the paper media above.
Materials:
Equipment Needed:
- Glass capillary tubes, spotting racks and clips
- 6 x 15 cm sheets of Whatman #1 paper
- 11 cm Whatman #1 circular filter papers
- Cellulose TLC sheets (Eastman or ?) cut to 10 x 10 cm squares
- Station of chicken paper supplied with hole punch, 15 cm ruler, drawing
compass, white rug yarn, flattened round wire hairpins and Cub® stapler
- Spraying station in hood with hair drier; an oven at 100°C
- 250 mL beakers, watch glasses, 10 cm diameter, glass petri dishes
Reagents Needed: (Go back to cylindrical chromatography
procedures.)
CAUTION: Solvents are flammable! Containers should be
capped and safely separated from open flames.
These two solvents work well for amino acid separation:1
- Solvent A = 250 mL 1-butanol (n-Bu): 250 mL tertiary butanol (t-Bu):
100 mL conc. acetic acid (HAc): 200 mL distilled water (dH2O)(5:5:2:4)
and
- Solvent B = 250 mL n-Bu: 250 mL t-Bu: 150 mL concentrated ammonia (NH3):
150 mL distilled H2O (5:5:3:3). Warm to room temp. and mix thoroughly
before use.
0.2% ninhydrin - 2.0 g ninhydrin in 1 L 95% ethanol - store @ 5°C
in dark bottle. 0.01% collidine may be added to improve stability.
Amino acid standards and samples: dissolve 0.010 g of each amino acid
in 10 mL 0.10 M HCl (or 1.0 mM Na2EDTA) = 1 mg/mL Lysine, Proline,
Glycine, Aspartate, Tyrosine and Phenylalanine.
1Helser, T.L. (1990) "Amino Acid Chromatography: The
'Best' Technique for Student Labs." J. Chem. Educ., 67, #11, 964-966.
Would you like
to go to the top of this page, to my home
page, or back to the lab
schedule?
If you have questions or comments, write the:
Author of this page: Terry
Helser - helsertl@oneonta.edu
Web Coordinator: Steve
Maniscalco - maniscsj@oneonta.edu
Or return to the SUNY @ Oneonta Home
Page to see where we live and work.
Last Modified on 8/26/97