 |
Terry L. HELSER, Ph.D.
Professor
Chemistry Department
S.U.N.Y. College at Oneonta,
Oneonta, N.Y. 13820-4015
Phone: (607)436-3518, or -3193;
Fax: (607)436-2654
|
This appendix contains useful information on the metric
system, pH and logarithms, and
instructions on the care and use of pH meters and
spectrophotometers.
You can also go back to my home
page or jump to the addresses at the bottom.
THE METRIC SYSTEM
Linear Measure
|
1 mµ (10-6 mm) =
|
0.000000039 in
|
|
1000 mµ =
|
1 micron (10-3 mm) =
|
0.000039 inch
|
|
1000 µ =
|
1 millimeter =
|
0.039 inch
|
|
10 mm =
|
1 centimeter =
|
0.393 inch
|
|
10 cm =
|
1 decimeter =
|
3.937 inches
|
|
10 dm =
|
1 meter =
|
39.37 inches
|
|
1000 m =
|
1 kilometer = 1 km =
|
0.62137 miles
|
Weight Measure
|
1 microgram (10-6g) =
|
0.000000035274 ounce
|
|
1000 µg =
|
1 milligram (10-3g) =
|
0.000035274 ounce
|
|
1000 mg =
|
1 gram =
|
0.035274 ounce
|
|
100 gms =
|
1 hectogram =
|
3.5274 ounces
|
|
1000 gms =
|
1 kilogram = 1 kg =
|
2.204 pounds
|
|
28.35 gms =
|
1 ounce
|
|
453.59 gms =
|
1 pound
|
|
907.18 kilograms =
|
1 ton
|
Liquid Measure
|
1 microliter (10-6L) =
|
0.0000338 fl. ounce
|
|
1000 µL =
|
1 milliliter (10-3L) =
|
0.0338 fl. ounce
|
|
1000 mL =
|
1 liter = 1.0567 quarts =
|
0.26418 gallons
|
|
0.47 liter =
|
1 pint
|
|
0.95 liter =
|
1 quart
|
|
3.79 liters =
|
1 gallon
|
Reference: L. J. Hale (1965) Biological Laboratory Data,
"Methuen's Monographs," John Wiley & Sons, New York, 147 pp.
You can go back to the top, to the
pH
meter instructions or the closing from here.
pH and Logarithms
Water ionizes slightly: H2O(H2O)n <--->H+(H2O)n
+ OH-. Direct measurements have determined that it is 0.00001%
ionized at room temperature, so the concentration of each of the ions in
pure water is 1 x 10-7 M. As you may have observed, the molar
concentration of hydrogen ions (symbolized as [H+]) in water
solutions is usually a very small number, indicated by negative powers
of 10 (like 10-7 M = 1/10,000,000 M = 0.0000001 M). To avoid
the use of such numbers, scientists defined a pH scale as the
exponent of 10 that gives one over the molar concentration of hydrogen
ions,
or: pH = log 1/ [H+] = - log [H+]. Thus, for pure
water:
pH = log 1/10-7 = - log 10-7 = -(-7) = 7.
Since acids increase the [H+], the pH is less than 7. If
the pH is greater than 7, a solution is basic.
1 <-- Acidic <--7 --> Basic --> 14 = pH scale.
The following is a review of logarithms which should
help you understand and use the pH scale. The logarithm of any number is
the exponent ("power") of 10 (called a "base") that gives the number.
Thus: log 100 = 2, since 102 = 100, and
log 3.16 = 0.5, since 100.5 = 3.16.
The following relationships may help:
|
100 = 1
|
log 1 = 0
|
|
log (a x b) =
|
log a + log b
|
|
101 = 10
|
log 10 = 1
|
|
log a/b =
|
log a - log b
|
|
103 = 1000
|
log 1000 = 3
|
|
log 1/a =
|
- log a
|
|
10-1 = 0.1 = 1/10
|
log 0.1 = -1
|
|
log ab =
|
b(log a)
|
|
10-3 = 0.001 = 1/103
|
log 0.001 = -3
|
|
|
|
In order to find the log of a number, convert it to scientific notation.
-
For example: log 250 = log (2.5 x 102) = log 2.5 + log 102
(look up 2,5,0 under N in a log table, or hit 2, 5, 0 and "log" keys
on your calculator)
= 0.398 + 2 = 2.398,
or log 0.0025 = log (2.5 x 10-3) = log 2.5 + log 10-3
= 0.398 + (-3) = -2.602
To find the number whose log is 6.8, separate the exponent into two, as
follows:
106.8 = 100.8 + 106, then look up
8000 in the body of the table to give N = 6,3,1, so 6.8 = log 6.31 + log
106 = log (6.3 x 106).
Most calculators allow you to type "6.8", "2nd Function", "Log" or "6.8",
"Inverse Log" to get the answer "6,300,000" or "6.3 e6", meaning 6.3 x
10 to the exponent 6.
To find the number ( [H+] ) whose log is -7.2 (as in, "The pH
= 7.2, find the [H+]."), first realize that if 7.2 = pH = -
log [H+], then [H+] = antilog or inverse log of -7.2
(multiply through by the minus).
To solve this, add and subtract the next whole number exponent as follows:
-7.2 + 8 - 8 = 0.8 + (-8), which = log (100.8 x 10-8
M). Look up 8000 in a table, which gives the Number 6,3,1. So, -7.2
= log 6.31 + log 10-8 = log (6.3 x 10-8 M). Thus,
6.3 x 10-8 M = [H+] that gives a pH of 7.2.
You can jump back to
the top, to pH and Logarithms,
to spectrophotometers or the closing
here.
pH Meters
Care and Use
Before using a pH meter, the electrodes must be washed thoroughly with
distilled water and blotted gently with tissue. The test and standard solutions
should be the same temperature, since the pH of a buffer is temperature-dependent.
For example, a common buffer has a pH of 7.0 ± 0.02 at 25°C,
but at 0°C the pH is 7.12 ± 0.02. Other common
errors in probe use are given below.
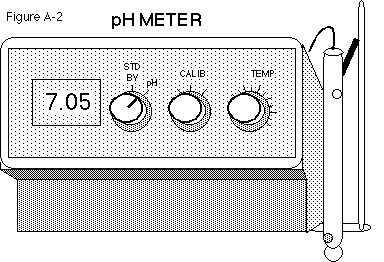
To use the meter:
-
Immerse the electrode(s) into the standard buffer solution and switch the
left knob from stand by to pH (see Figure A-2).
-
Adjust the meter with the calibration knob to the known pH of the buffer
at that temperature.
-
Rinse the electrode(s) and check the operation of the pH meter with another
standard buffer, for example, a buffer of pH 4 or pH 10. Use the temperature
knob to adjust, if necessary.
-
Repeat steps 1-3 until the readings are stable.
 Ideally,
the pH of each of the pair of buffers selected to calibrate the meter should
bracket the expected pH of the test solution. If the pH of the second buffer
is within 0.05 pH of the expected value, the pH meter is working properly.
Ideally,
the pH of each of the pair of buffers selected to calibrate the meter should
bracket the expected pH of the test solution. If the pH of the second buffer
is within 0.05 pH of the expected value, the pH meter is working properly.
The use of two standard buffers of different pH is the only reliable
way of checking a pH meter. Since the characteristics of the glass electrode
change with time, the calibration of the meter must be checked at regular
intervals.
Once the meter is properly calibrated, the pH of the test solution may
be determined. After use, rinse the electrode with distilled water. Since
the glass tip of the electrode is very sensitive to dehydration, keep it
immersed in distilled water when not in use.
You can go back to the top, to pH
and Logarithms, or the closing from here.
Errors in pH Measurement
The Sodium Error: Most pH-responsive glasses are somewhat sensitive
to Na+. In fact, glass electrodes have been prepared which are
more sensitive to alkaline earth cations than they are to H+
(the so-called cation electrodes). At high pH as little as 0.1 M
Na+ can cause an apparent pH decrease of 0.4 unit. A curve for
the correction of the sodium error is provided by the manufacturers of
glass electrodes. This problem may be alleviated by the use of potassium
salts rather than sodium salts, or by the use of the newer glass electrodes
with reduced sensitivity to Na+.
Temperature and Ionic Strength: The dissociation of acids and
bases is temperature- dependent. Thus, the pH of a buffer solution can
change dramatically with temperature. For example, tris(hydroxymethyl)
aminomethane (Tris), a very commonly used buffer in biochemical research,
has a dissociation constant which is strongly temperature-dependent.
The activity coefficients of the charged species of a buffer are also
affected by the ionic strength. Thus, addition of a salt at concentrations
exceeding 0.025 M to a buffer solution can have profound effects on the
pH. Further, dilution of a buffer will also generally have an effect on
the pH. Thus, the pH of the final solution should be determined directly
before it is used.
Fouling of the Electrodes by Biological Samples: A pronounced
drift in the pH of a solution may sometimes be noticed. In some cases,
this drift may be caused by a small crack in the electrode; in other cases,
however, it may be caused by the deposition of a thin coating of protein
on the glass membrane. This coating may be removed by rinsing the electrode
in a strong detergent solution.
If the pH of samples containing suspended materials is determined, the
fiber junction of the calomel electrode can readily become plugged. Since
the fiber junction forms the salt bridge with the solution, it is imperative
that it be clean. A plugged junction may be cleaned by briefly immersing
the tip of the electrode in a solution of warm, concentrated nitric acid.
References:
l. Bates, R.G., Determination of pH: Theory and Practice, Wiley,
New York, 1964.
2. Dole, M. The Glass Electrode, Wiley, New York, 1941.
From: Wharton, D.C. and R.E. McCarty (1972) ExperimentsandMethods
in Biochemistry, Macmillan Publishing Co., Inc., New York,
N.Y.
You can go back to the
top, to pH and Logarithms, the
pH
meter or the closing from here.
Spectrophotometer Use
INTRODUCTION
All compounds absorb specific types of electromagnetic radiation, with
visible light being just one region of this spectrum. Some of the most
important optical techniques in biochemistry depend upon this fact. For
example, spectrophotometers that measure light absorption are used for
quantitative work, since absorbance is proportional to the concentration
of the absorbing solute. Thus, the course of a chemical reaction can readily
be followed by using a color of light (wavelength) that is strongly absorbed
by one of the products but not by any of the reactants. A simple instrument,
the Spectronic 20, is described below.
The Optical System
The operation of this instrument is shown in the simplified diagram
in Figure A-1.
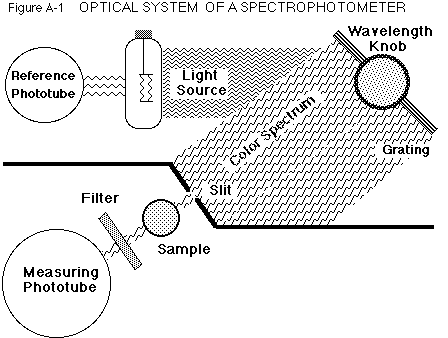 White
light from the tungsten lamp is directed onto a reference phototube and
a diffraction grating, which acts like a prism and spreads the light into
a spectrum. Setting the wavelength sweeps the spectrum over the slit until
only the selected color passes through and illuminates the sample in the
chamber.
White
light from the tungsten lamp is directed onto a reference phototube and
a diffraction grating, which acts like a prism and spreads the light into
a spectrum. Setting the wavelength sweeps the spectrum over the slit until
only the selected color passes through and illuminates the sample in the
chamber.
Light which is not absorbed may pass through a filter (needed for the
red region) before hitting the measuring phototube, where light is converted
into an electrical signal. This is adjusted for variations in the lamp
intensity by the reference signal, and then registered on the meter as
absorbance and transmittance.
Whenever the sample is removed from the chamber, an occluder automatically
falls into the light path so that a zero light level may be set on the
meter. A circuit operated with the right, front knob alters the lamp so
zero absorbance can be set with a blank solution in the chamber.
Operating Procedure
-
Turn the power on. Turn the left knob on the front of the case clockwise
until the meter needle is in the center of the scale.
-
Allow to stabilize for at least 5 minutes before use.
-
Select the wavelength. Turn the knob on the top, right of the case until
the correct number is opposite the pointer visible in the window beside
the knob.
-
Zero the meter. Without a test tube in the chamber, adjust the left,
front knob until the meter needle indicates infinite absorbance (= 0 transmittance).
The sample chamber cover must be closed.
-
Adjust for maximum absorbance. Fill a cuvette (test tube) with the blank
solution (4 mL minimum), wipe the outside, align in the chamber, and close
the cover. Adjust the right, front knob until the meter reads zero absorbance
(= 100% transmittance).
-
Measure sample absorbance. Fill the same cuvette, or one matched with the
blank cuvette, with the sample solution. Wipe, align in the chamber, close
the cover, and read the absorbance (or transmittance, if desired) from
the meter.
-
Guard against parallax errors by ensuring only one needle is visible
in the mirror before taking the reading.
Important:
The meter must be adjusted for maximum absorbance (step 4.)
each time the wavelength is changed. It is also necessary to occasionally
check for meter drift from zero absorbance (step 3.).
That's all for now. Again, you can jump to the
beginning,
to my home
page or the biochemistry
or non-major's
chemistry pages.
If you have questions or comments, write the:
Author of this page: Terry
Helser - helsertl@oneonta.edu
Web Coordinator: Steve
Maniscalco - maniscsj@oneonta.edu
Or return to the SUNY @ Oneonta Home
Page to see where we live and work.
Last Modified on 8/14/01


White
light from the tungsten lamp is directed onto a reference phototube and
a diffraction grating, which acts like a prism and spreads the light into
a spectrum. Setting the wavelength sweeps the spectrum over the slit until
only the selected color passes through and illuminates the sample in the
chamber.