 ImmunoAssay
ImmunoAssay
Detection of Meat Adulteration
This page gives some theory about Immunology,
precipitin
reactions and the Ochterlony test. Procedures
needed to test for the adulteration of cow, pig or horse meat follow. First,
ensure that you have the materials and reagents
needed for these assays. You can also jump to other lab
procedures in an appendix, the biochemistry lab
schedule, my home
page or the addresses at the bottom.
Multicellular organisms must defend
themselves against attack by a host of pathogens, including viruses, bacteria
and parasites. In addition to nonspecific mechanisms such as phagocytosis
shared with lower animals, vertebrates have evolved an acquired immune
response, which provides protection against pathogens after the first
exposure. For example, we rarely contract chicken pox after the first illness
as a child. This immunity
-
is highly specific (immunity to chicken pox does not protect against
other viruses, even other pox viruses),
-
involves a form of cellular memory, since protection lasts for years,
and
-
can distinguish between "self" and "non-self"
since you normally
don't respond to your own proteins, but do against another person's or
animal's.
Tremendous strides have been made recently in understanding what is now
known to be a very complex system. It can be divided into at least two
major components, cell-mediated and humoral immunity. The
first is produced by white blood cells called T-lymphocytes, now
known to include several kinds with different functions. Humoral immunity
results from B-lymphocytes, which produce freely circulating proteins
called antibodies (anti-foreign bodies). These specifically
bind to parts of molecules or cells, termed antigens (since these
generate
antibodies).
It is this antigen-antibody reaction that is the focus of numerous specific
tests that have become indispensable in research and medicine.
You can go back
to the top or the closing from here.
PRECIPITIN REACTIONS:
When an antigen is mixed with the correct concentration of its specific
antibody, a precipitate forms. This is a latticework of antigen molecules
bound together by antibody molecules, which each have two (divalent)
or more (polyvalent) binding sites for a single part of the antigen's
surface. Thus, each antibody can bind to two or more antigens at the same
time. In addition, each antigen usually has several regions of unique structure
(called antigenic determinants), each of which can induce production
of a specific antibody to bind to it. Thus, each antibody preparation is
usually a mixture of different antibodies, each specific for a different
part of the antigen, as indicated in figure 1. below.
Also, the presence or absence and the amount of precipitate which results
when mixing antigen with antibody (the precipitin reaction) is dependent
on the relative concentrations of each. Thus, in a large excess of antibody,
each will bind to only one antigen site at a time, on average, forming
soluble complexes. At equivalence, each antibody molecule will tend
to link the maximum number of antigens to produce the maximum precipitate
possible. In large antigen excess, available antibody will be quickly
bound to two (or the valence number of) antigens, such that small, soluble
complexes again predominate (see Fig. 1.).
Figure 1. Antigen-Antibody Complexes
|
a. Antibody Excess
|
b. Equivalence
|
c. Antigen Excess
|
You can go back to
the top or the closing from here.
Ouchterlony Double Diffusion Test:
In 1948 S.D. Elek and O. Ouchterlony independently described a test
based on the precipitin reaction, in which antigen and antibody diffuse
toward each other from separate wells cut in agar. When they meet in optimal
concentration, a precipitate forms in a band between the wells, but only
if the antibody is specific for that antigen. Often the line is offset
toward one well and curved rather than straight. This indicates that either
the antigen (Ag) or antibody (Ab) has diffused more rapidly
than the other. While a number of causes can produce this result, it is
usually based on molecular size, with the smaller molecule producing a
line curved away from and more distant from its own well (Figure 2.).
Figure 2. Ag Size << Ab Size
If there are multiple components in either preparation, multiple bands
can form due to the different diffusion rates of molecules of different
sizes. Thus, this test can be used to check purity. In addition, by placing
different antigen preparations in neighboring wells equidistant from the
antibody well, this assay can test for identity, partial identity, or nonidentity
of the antigens with respect to the antibody used. If the same Ag
is present in adjacent wells, a continuous precipitin line forms (Fig.
3.a). If the Ag mixtures contain the same Ag and also a different one,
a spur forms (Fig. 3.b). Different Ags produce crossing lines (Fig.
3.c).
Figure 3. Identity Patterns in Ouchterlony Tests

|
a. Identity
|
b. Partial Identity
|
c. Nonidentity
|
Thus, this or similar tests are extremely valuable for determining the
purity of cell fractions and for indicating the identity or proving the
lack of identity between two isolates. It should be stated that an identity
pattern does not necessarily prove molecular identity, since different
antigens may share the same antigenic determinant (partial surface shape)
recognized by the antibody used.
You can go back
to the top, to the assay procedure or the
closing
from here.
An Ouchterlony Double Diffusion Test can be used
to demonstrate Ag/Ab precipitin reactions and immune specificity by testing
the animal source(s) of ground meat samples as given below.
Equipment Needed:
Immunodetective Biokit® - #20-2100, Carolina Biol. Supply Co. 2700
York Rd., Burlington, NC 27215. Refrigerate sera, dyes, chemicals until
day of use.
Pour agar plates 24-48 hours prior to lab - loosen caps on agar bottles,
melt (microwave on 50%) in boiling water, cool to 50-60°C and pour
just enough to cover each section?s bottom. Leave lids ajar (in sterile
hood?) until set; invert until used.
Set up a station with clean paper (Chicken paper in hoods?) for dyes, salts,
antigens and antibodies.
4 well cutters (3 mm OD - Fisher #02-678, pk of 10) in tubing to vacuum
traps and source
5, 10 & 20 µL Drummond WireTrole® pipets, #W-051,
W-101 & W-201
from Drummond Scientific Co., Broomall, PA 19008
microtiter or spot plates
mortar and pestle, screw capped vials for meat samples
To replace parts of the kit, purchase and prepare:
Serum Antigen Set - #20-2101, Carolina Biol. Supply Co. 2700 York Rd.,
Burlington, NC 27215
Serum Antibody Set - #20-2102, Carolina Biol. Supply Co. 2700 York Rd.,
Burlington, NC 27215 (Individual Ag/Ab pairs also available, #2105,6 or7)
Albumin, Bovine - Sigma #A 7030, 5 g; make 10 mL of 1 mg/mL in PBS and
filter sterilize, if possible.
Antibodies from Research Products, Miles Labs, Elkart, IN 46514; US biochemical
Corp., P. O. Box 22400, Cleveland, OH 44122; or Cappel Labs, Cochranville,
PA 19330: Anti-Albumin, Bovine (Rabbit Ab) - Miles #65-111-1, USB # 1100A
or Cappel #0102-0342; Anti-Albumin, Horse or Swine (ask).
To prepare, add 2 mL sterile dH2O, avoid foam!
LE Agarose, 100 g - #50002, FMC Corp., Marine Colloids Division, 5 Maple
St., Rockland, ME 04841
Reagents Needed:
PBS Buffer (Phosphate Buffered Saline) - 3.52 g NaCl (0.15 M), 0.2 g NaN3
(0.05%), 0.4 g Na2HPO4 (20 mM), 0.392 g KH2PO4
dissolved in 400 mL dH2O, autoclaved 15 min.
16 Agarose Plates: in a 500 mL flask, add 3.6 g LE Agarose (1.5% final)
in 240 mL PBS, autoclave and pour plates as above.
5 g samples of ground beef, pork or ?? - must be fresh (uncooked, frozen
is fine)
You can go back
to the top or the closing from here.
Procedure:
-
Obtain a solid, 1.6% agarose plate and well cutter. Attach the tubing from
the side arm of the vacuum flask to a source and sterilize the cutter with
flame and/or 70% ethanol.
-
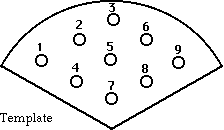
Using a gentle vacuum, cut the number and arrangement of wells into the
agar sector(s) as needed using the template below (Fig. 4).
Keep the plate covered as much as possible to minimize contamination
and be certain you have removed the cut agar plugs before trying
to fill the wells.
-
Fill the wells with dyes, salt solutions or Ags and Abs using 10 or 20
µL Wiretrol® capillary pipets.
-
For example, wells 2 and 6 might be filled with the dyes to demonstrate
double diffusion, and then wells 4 and 8 filled with the salt solutions
to show where BaSO4 precipitates. These results can be seen
after about 45 minutes to 1 hour.
-
To test control albumins (beef, pig and horse, for example) against the
antibodies to ensure they are all active, one could put the Ags in wells
1, 5 and 9 or 3, 5 and 7, and the Abs in 2, 4, 6 and/or 8.
-
To test the effect of dilution on precipitin line shape and position, one
could dilute the Ag (bovine albumin) by 1/1 to 1/10 or more with phosphate
buffered saline (PBS) and fill wells 2 through 8, leaving the Ab in well
5. Alternatively, dilute the Ab and test against Ag in well 5.
Work quickly and be careful not to tip or jar any solutions out
of the wells. You may be asked to use only 10 µLof each to conserve
supplies (note this for your report).
-
To test your own meat for adulteration, bring about 5 grams (thumb-tip
size) of raw, ground meat to the lab. Check the package label carefully
for terms like "Ground Meat," or "Ground Beef," and record this for your
report (these terms have specific definitions in USDA regulations).
-
Mash the sample in about 5 mL of PBS and drain the fluid into a vial to
use as the Ag sample.
-
To test various meat extracts or "Mystery Meat" samples provided, one could
fill wells 2, 5 and 8 with these and/or control Ags, and fill 3, 6 and
9 with the Abs and repeat the Abs in wells 7, 4 and 1. Thus, wells 3 and
7, 4 and 6, and 1 and 9 each contain the same Ab. This subjects all three
Ags to all three Abs in one sector. Be aware that secondary interactions
are probable with such multiple testing.
The precipitin lines take about 16+ hours to develop, and may disappear
after about 48 hours as the Ag/Ab ratio changes due to continued diffusion.
Thus, you should seal the plates with Parafilm® or tape
and record the results tomorrow. The precipitin lines will last much longer
if the plate is refrigerated after development.
Figure 4. Template for cutting equidistant wells in agar plates
Questions to help analyze the results:
-
With which Ab did the standard Ag (bovine albumin) react? With which did
it not react? How does this support or refute the idea of immune
specificity?
-
Discuss the fact that a goat can produce Abs to serum albumins of other
animals but not its own.
-
How might this test be used clinically, by the Red Cross faced with AIDS,
etc. in the blood supply, and by the US Department of Agriculture?
-
Which protein is larger, the antigen or antibody? What evidence supports
this?
-
What pattern (see Fig. 3) resulted between each set of wells? What does
this mean?
-
Did dilution of the Ag or Ab affect the position or shape of the precipitin
band? Explain.
-
Which animals were the source of each meat sample tested?
-
Antigen from which animals were not present in each sample?
-
Is there evidence of adulteration in any sample? Explain.
-
Could the meats be adulterated and still escape detection? How?
-
If a meat sample produced no precipitin line (did any?), what animals could
not
be the meat source?
-
Why is raw rather than cooked meat required for this test?
Reference: Small, P.A. III, Small, P.M. & Small,
P.A. Jr. (1976) "Understanding Immunology" Carolina Biol. Supply Co., Burlington,
N.C.
That's all for now. Again, you can
jump to the beginning, to my home
page or the biochemistry
or non-major's
chemistry pages.
If you have questions or comments, write the:
Author of this page: Terry
Helser - helsertl@oneonta.edu
Web Coordinator: Steve
Maniscalco - maniscsj@oneonta.edu
Or return to the SUNY @ Oneonta Home
Page to see where we live and work.
Last Modified on 8/14/01

