 Introductory
Biochemistry (Chemistry 330)
Introductory
Biochemistry (Chemistry 330)
Vitamin C
This page contains some theory on vitamins, particularly
ascorbic acid (vitamin C), questions to guide
experiments and a procedure and the materials
for determining its concentration in fluid extracts. An appendix
is available with help on pH, logs and the pH
meter. You can also leave me a message, go to
my home page,
or go back to the lab
schedule.
Vitamins are essential carbon compounds which heterotrophs
must obtain from their diets, though only in minute amounts because they
are cofactors for enzymes. Occasionally an animal can synthesize part of
its requirement (Vitamin D in man), and often symbiotic organisms provide
part or all of the requirement (the B vitamins by gut microbes).
There is no such thing as a general vitamin, but only ones for specific
species. Ascorbic acid (vitamin C) is required for only a few mammals like
guinea pigs, fruit bats and man. Deficiency causes scurvy, a disease
where the structural protein collagen can't be finished, and sufferers
literally fall apart. In addition, large doses of 0.25 g or more a day
have been shown to alleviate the symptoms of the "common" cold. Whether
such doses help prevent cancer and other diseases is still debated,1
and there is a risk of kidney and bladder problems because vitamin C is
acidic and most of it is excreted.
Assays often use the fact that ascorbic acid is an "anti-oxidant," (that
is, a reducing agent) which may cause its reported health benefits. It
is readily oxidized by the dye 2,6-dichloroindophenol (DCP). The reaction
is:
|
C6H8O6 +
|
DCP (Blue)
|
---->
|
C6H6O6 +
|
H2DCP (Colorless)
|
|
(L-ascorbic acid)
|
(= Oxidized)
|
|
(L-dehydroascorbic acid)
|
(= Reduced)
|
With this reaction, one can assay various foods, serum or urine for
vitamin C content, or determine the best methods of food preparation to
preserve it. For example, if you want to test for excretion of excess vitamin
C, you may have to arrange to take urine samples and store them in a stable
form both before and after taking supplements. Whatever you choose to investigate,
you need to prepare a standard curve and use it to determine vitamin C
(actually, all similar reducing agents) concentrations in your samples.
Would you like to go to the top
of this page, to my home
page, or back to the lab
schedule?
You must have your experiments planned and approved before
coming to the lab. Check with your instructor if you have problems. Suggestions2,
3 for study questions include:
-
Is the vitamin content the same in fresh, frozen and canned juices?
-
Does vitamin C content vary in different parts of a fruit or vegetable?
-
Do you excrete most of the vitamin C you ingest?
-
Which sources are high (low) in vitamin C? Is the name "Hi-C" false advertising?
-
How much of each food you tested must you consume to prevent scurvy? (10
mg/day)
-
How much to meet your minimum daily requirement? (Women = 55 mg/day; Men
= 60 mg/day)
-
Does cooking destroy the vitamin C? Does the metal in the pan affect this?
-
Do raw, boiled, steamed , fried or baked vegetables retain more vitamin
C?
-
What methods of preparation and storage best preserve vitamin C content?
Would you like to go to the top
of this page, to my home
page, or back to the lab
schedule?
Prepare a flow diagram for your experiment and
submit it before the lab, as directed by your instructor. Include a photocopy
of one journal article you used. If you have questions, ask them before
coming to the lab!
Procedure: (Microscale Titration - Standard Curve)
Each student or team will need 2 or 3 Beral pipets, flat toothpicks and
a 24 well plate (Costar® or Falcon®).
-
Obtain about 20 mL of dye in a small, very clean beaker.
-
Obtain about 2 mL of each ascorbic acid standard (usually 1.0, 0.5, 0.25
and 0.125 mg/mL solutions in 1% oxalic acid) in small test tubes that are
very
clean and dry.
-
With a Beral pipet, carefully transfer 0.5 mL of the standard with the
lowest concentration into each of 3 wells out of the 6 on one side of the
plate.
-
With another Beral pipet filled with DCP, add drops of dye to a well of
sample while carefully counting drops and stirring with a toothpick. The
end point is when the dye stops becoming colorless and stays a light pink
for at least 30 seconds.
-
Repeat the titration for each of the other wells, and for the other standards
available, in order of increasing vitamin C concentration. See the figure
below.
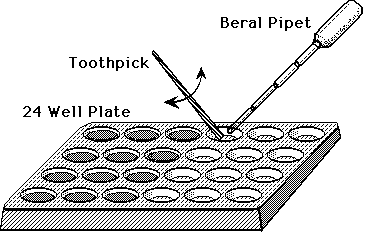
-
Plot the average drops of DCP used (ordinate = Y-axis) against the concentration
of vitamin C in mg/mL.
With this plot you can find the concentration of ascorbic acid in your
food or urine samples. Just determine the average number of drops of DCP
required to oxidize a 0.5 mL sample. Locate this value on the Y-axis and
find the vitamin C concentration to which it corresponds on the X-axis.
Would you like to go to the top of
this page, to my home
page, or back to the lab
schedule?
Materials
Each team will need 2 or 3 Beral pipets, flat toothpicks and a 24 well
plate (Costar® or Falcon®). To prepare their own standards from
vitamin C tablets, teams will need appropriate volumetric flasks to prepare
dilutions. Each team may also need a mortar and pestle to extract juices.3
Reagents
-
1% oxalic acid: Dissolve 5 g (Sigma #O-0505, oxalic acid dihydrate, ACS
Reagent) in 500 mL of distilled or deionized water.
-
DCP: Dissolve 0.200 g 2,6-dichloroindophenol (DCP; Eastman #3463) in 0.5
L of distilled or deionized water. This is enough for two lab sections.
Store refrigerated, if possible.4
-
1 mg/mL ascorbic acid: Dissolve 0.100 g L-(+)-ascorbic acid (Eastman #4640)
in 1% oxalic acid and dilute to 100 mL in a volumetric flask.4
-
Or, dissolve a 100 mg tablet in water, filter to remove debris and add
1% oxalic acid.
-
Dilute 1:1 with 1% oxalic acid to obtain 0.5, 0.25 and 0.125 mg/mL solutions.
50 or 100 mL of each dilution is enough for a lab section.
These solutions can be used for at least a week if stored in a refrigerator.3
-
Clean Up: Swish the plate and pipets in hot, soapy water;
then rinse thoroughly in tap water and deionized water (3 times each).
Return materials where you found them.
Literature Cited
1. Pauling, L. Vitamin C and the Common Cold; Freeman: San Francisco,
1970.
2. Thompson, S. ChemTrek; Allyn & Bacon: Boston, 1990; p
211.
3. Johnson, E.R. J. Chem. Educ. 1988, 65, #10, 926-927.
4. Helser, T. L. J. Chem. Educ. 1995, 72, #1, A10-11.
That's all for now. Again, you can jump to the
beginning,
to my home
page or the biochemistry
or non-major's
chemistry pages.
If you have questions or comments, write the:
Author of this page: Terry
Helser - helsertl@oneonta.edu
Web Coordinator: Steve
Maniscalco - maniscsj@oneonta.edu
Or return to the SUNY @ Oneonta Home
Page to see where we live and work.
Last Modified on 8/14/01


