1. Draw Lewis structures of the molecules below. Skeleton structures have been provided. You will ne ed to complete the process by
-> adding up the number of valence electrons,
-> distributing those electrons on the structure in an appropriate way, and
-> calculating formal charges.
Be sure to show all unshared electrons. In some cases there will be more than one reasonable Lewis structure; in these cases the existence of resonance will be indicated.
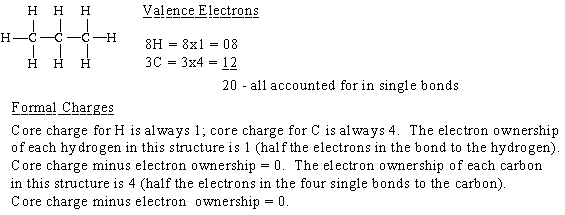
(a) propane,

From this point on the calculation of formal charge will be shown only if the result is not zero.
Propane is an alkane. An alkane is a hydrocarbon in which there are no multiple bonds. A hydrocarbon is a c ompound which consists of only hydrogen and carbon atoms.
(b) propene,
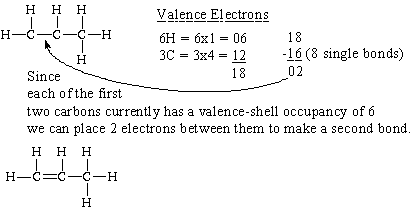
Propene is an alkene. Alkenes are hydrocarbons that have a double bond.
(c) propyne,
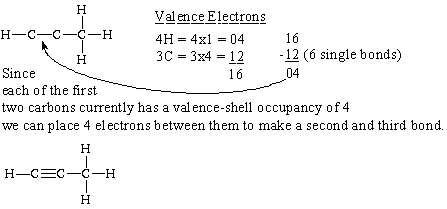
Propyne is an alkyne. Alkynes are hydrocarbons that have a triple bond.
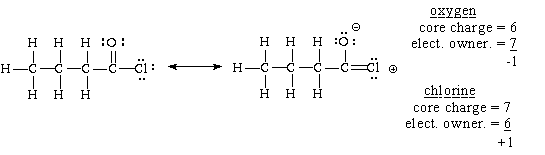
(d) butyryl chloride,

(Two resonance structures; the one which involves charge separation does not make a large contribution.)
If we accommodate two of the remaining 12 valence electrons between the oxygen and carbon to which it is attached, forming a second bo nd there, then 4 electrons can reside, unshared, on the oxygen, and six, unshared, on the chlorine. This takes care of all 12.
Alternatively, if we accommodate two of the remaining 12 valence electrons between the chlorine and carbon to which it is attached, forming a second bond there, then 4 electrons can reside, unshared, on the chlorine, and six, unshared, on the oxygen. This takes care of all 12. The calculations of formal charge for the oxygen and chlorin e in the structure on the right are shown below.
The two alternatives are the resonance structures for this compound. Since the first one does not involve charge separation and the second does, the first is the greater contributor, i.e. the molecule looks more like the first than the second.
(e) carbon tetrachloride,

Carbon tetrachloride used to be used as a "dry cleaning" spot remover. It is no longer used for this purpose because it has been found to be very toxic.
(f) ethanol,
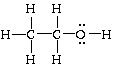
In this case there are 20 valence electrons: 6 from 6 hydrogens, 8 from 2 carbons, and 6 from oxygen. The single bonds account for 16 of them; the remaining two pair are accomm odated, unshared, on oxygen.
Ethanol, CH3CH2OH, is also called ethyl alcohol and it is the second member of the class of compounds known as alcohols. The functional group for alcohols is the -O-H group. Ethanol is also known as grain alcohol because it can be obtained from the fermentation of grains (rye, corn etc.) which contain starch. Ethanol is the alcohol found in alcoholic beverages. Methanol, CH3OH, is the first me mber of the alcohol family. It is also called wood alcohol because it can be obtained from wood. It is toxic.
(g) diethyl ether,

In this case there are 32 valence electrons; 10 from the 10 hydrogens, 16 from the 4 carbons, and 6 from th e oxygen. Twenty-eight of the 32 are accounted for by the 14 single bonds. Since all the carbons have a valence-shell occupancy of 8 at this point, no more bonds can be formed. Consequently, the remaining four electrons are accommodated on the oxygen, unshared.
Diethyl ether is the "ether" which was employed for many years as a general anesthetic. Owing to its great flammability it has been supplanted by other compounds.
[Note: It is possible and, for convenience, in many cases desirable to write complete or incomplete Lewis structures in condensed form. Thus, for diethyl ether we could write CH3 - CH2 - O - CH2 - CH3 or even CH3CH2 - O - CH2CH3. Notice that in condensed form, not all of the bonds are explicitly shown; their existence is implied. The location of the bonds must be determined from the way atoms are grouped in the structure and from the usual valency rules -- for example, carbon usually carries four bonds. We shall begin to use this condensed notation in the remaining exercises.]
(h) cadaverine,
![]()
There are 44 valence electrons in this case; 14 from the 14 hydrogens, 20 from the 5 carbons, and 10 fro m the 2 nitrogens. Single bonds account for 40 of these. Since all the carbons have a valence-shell occupancy of 8 at this point, no additional bonds can be formed. Consequently, the remaining 4 electrons are accommodated on the two nitrogens -- two unshared on each nitrogen.
Cadaverine is an amine. Amines are compounds that have a nitrogen which is joined to a carbon containing group. The nitrogen may be joined to other carbon containing groups or to hydrogens. Cadaverine is formed through the action of the comma bacillus on protein containing materials, eg, it is found in rotting meat; hence, its name.
(i) acetic acid,
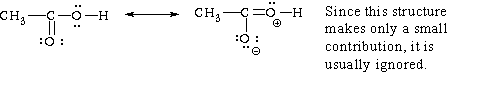
There are 24 valence electrons in this case: 4 from the 4 hydrogens, 8 from t
he carbons, and 12
from the oxygens. Fourteen electrons are accounted for in the 7 single bonds in the skeleton
structure given, leaving 10. In this case a second bond can be formed between the central carbon
and either of the oxygens. This leads to two resonance structures, although, as noted above, one
of them is a minor contributor. The second carbon oxygen bond uses two of the remaining 10
electrons, leaving 8. These 8 are accommodated as unshared electrons on the oxygens, as shown
above.
Oxygen always has a core charge of +6. The electron ownership of the top oxygen in this right hand resonance structure is 5. The formal charge is 6-5 = +1. The bottom oxygen has an electron ownership of 7. Its formal charge is 6-7 = -1.
Acetic acid is an example of a carboxylic acid. These acids are characterized by the carboxyl, -COOH, functional group. Vinegar is a 5% aqueous solution of acetic acid.
(j) acetaldehyde,
![]()
Acetaldehyde is an example of an aldehyde, having the -CHO functional group. It is an intermediate in the metabolism of ethanol to acetic acid. It is the acetaldehyde in a inebriated driver's breath that gives a positive "Breathalizer" test.
(k) benzene, (two resonance structures)
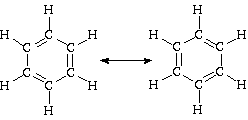
Be aware that no atoms were moved to get from one structure to the other; only electrons have been moved. One structure was NOT rotated by 60 degrees to get the other.
(l) diazomethane, (two resonance structures)
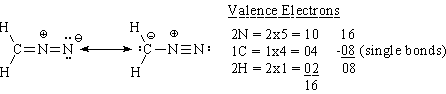
The remaining 8 valence electrons can be distributed in two ways, as shown. The resonance structure on the left would be expected to contribute a little more than the one on the left because in it N holds the negative charge, while in the other one the C holds the negative charge. Since N is more electronegative than C, it is more willing to accept negative charge, making the left structure more important.
2. Define: (a) isomers, (b) structural isomers.
(a) Isomers are compounds that are different from each other but have the same molecular formula.
(b) Structural isomers are compounds that have the same molecular formula but are different from each other by virtue of having different atomic connections.
3. For each pair of compounds below indicate whether or not they are structural isomers.

(a) No, the molecular formulas are different. (b) Yes, the molecular formulas are the same and the atomic connections are different. (c) No, these molecules are identical, not isomers. Th ey are drawn differently, but they are the same; as an analogy, if you took two pictures of a snake - one when the snake was stretched out and the other when it was coiled, it would still be the same snake.
3. Consider the compounds shown below.
(a) Are I and II structural isomers?
Yes -- same molecular formula, C4H10O, but different atomic connections.
(b) Are I and III structural isomers?
Yes -- same molecular formula, C4H10O, but different atomic connections.
(c) Are I and IV structural isomers?
No, these are the same compound. See 3(c), above.
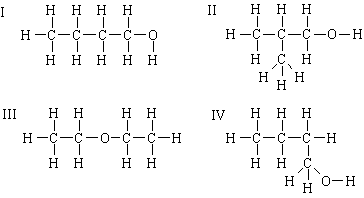
4. For each bond shown below indicate whether it would be non-polar covalent, polar covalent, or ionic; if polar indicate direction of dipole.
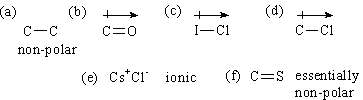
The following questions can be found at the end of chapter 3, on pages 62-64, in "The Extraordinary Chemistry of Ordinary Things," 3rd edition, by Carl H. Snyder.
5. #5.
(a)LiBr (b)CaS (c)Na2S (d)BeO (e)Cl2 (f)SiH4
6. #8.
(a) In the air.
(b) In a gold mine; around someone's neck; around someone's finger.
(c) In any living organism; in coal; in crude oil.
(d) Table salt; sea water.
(e) W ater.
(f) Several drugs are mentioned in the chapter in which this would be the case; also, proteins, not mentioned in the chapter.
7. #9.
(a) Nitrogen (b) Sulfur. (c) Hydrogen (d) Neon
(e)Atomic number = 2+8+2 = 12 = magnesium. (f) Hydrogen.
(g) Magnesium, again. (h) Atomic number = 2+3 = 5 = boron.
(i) Atomic number = 2+4 = 6 = carbon.
8. #10. The difference in electronegativ ity of the two elements is shown in each case below. If the difference is greater than 1.7 the bond will usually be ionic, otherwise covalent.
(a) 0.86 - covalent (b) 1.78 - an anomalous case; H-F is highly polar, but actually still covalent contrary to what we might predict. (c) 2.27 - ionic (d) 2.03 - ionic
(e) 0.4 - covalent (f) 0.35 - covalent (g) 1.68 - a borderline case, actually ionic
(h) 2.13 - ionic
9. #13.
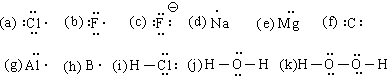
10. #14. Oxygen would have to lose its 6 valence electrons to attain the electronic structure of helium. This would result in a cation with a +6 charge. Such an ion would be very unstable. Single atom ions rarely carry more than 3 units of charge. This ties in with the statement that atoms that have fewer than 4 elec trons in their valence shell will lose or share them when forming bonds to other atoms and atoms with more than 4 will gain or share.
11. #19. Because they have the same number of valence electrons.
12. #29. It would be correct to write the empirical formula of each as CH. It would be incorrect to write the molecular formula of each as CH. The empirical formula provides the atom ratio in a compound, and it is 1:1 for both acetylene and benzene. The molecular formula tells us the number of atoms of each type in the compound. So, the molecular formula of acetylene is C2H2 and that of benzene is C6H6.
13. #33. Silicon is usually the victim in these tales. It is adjacent to carbon in group IVA of the periodic table. Like carbon it has four valence electrons. Of all the elements it is closest in properties to carbon.